Media Contact:
Marc Boston – LifeScan
mboston@lifescan.com
Results from New Study of Real-World Evidence Showing People with Type 2 Diabetes Achieved Clinically Significant Improved Glycemic Control with One to Two Blood Glucose Checks Per Day Using a Smart Blood Glucose Meter and Mobile App Published in Diabetes Therapy
The full publication is available with open access at: Diabetes Therapy, Sustained Improvements in Readings In-range Using an Advanced Bluetooth® Connected Blood Glucose Meter and a Mobile Diabetes App: Real-World Evidence from More than 55,000 People with Diabetes.
MALVERN, Pa., May 18, 2023 – LifeScan, a world leader in blood glucose monitoring, today announced that results from the study of real-world evidence Sustained Improvements in Readings In-Range Using an Advanced Bluetooth® Connected Blood Glucose Meter and a Mobile Diabetes App: Real-World Evidence from more than 55,000 People with Diabetes have been published in the peer-reviewed journal Diabetes Therapy. A smaller subset of these results was first published in the peer-reviewed journal Diabetes Technology and Therapeutics (DTT) in June, 2022. This new analysis focuses on changes over 180 days given that sustained outcomes for people with diabetes are more clinically relevant.
“Blood glucose meters are used by over 95% of people with diabetes who monitor their glucose, making these real-world findings particularly relevant to tens of millions of people who rely on blood glucose meters to make decisions every day and to their healthcare providers,” said study co-author, Dr. Elizabeth Holt, Head of Global Medical, Clinical, and Safety, LifeScan. “At the very least, the findings from this study should prompt people with either type 2 or type 1 diabetes to discuss with their healthcare provider if and how they could benefit more from using a smart blood glucose meter and its companion mobile app.”
Using real-world data from more than 55,000 people with diabetes (PWD), this study aimed to understand if using the OneTouch Reveal® (OTR) mobile app with the OneTouch Verio Reflect® (OTVR) meter – synced via Bluetooth® wireless technology – could support sustained glycemic improvements for 180 days in people with type 1 diabetes (PwT1D) or type 2 diabetes (PwT2D).
The real-world data demonstrated that PwT2D saw sustained, clinically significant improvements when engaging in just one to two OTR mobile app sessions per week, and performing one to two OTVR meter checks per day, over 180 days (n=3,563), including:
-
Reduced mean glucose by -20.0 mg/dL in PwT2D.
-
Improved glucose readings in range in PwT2D by +12.0 percentage points; and
-
Reduced hyperglycemic readings (>180 mg/dL) by -12.2 percentage points in PwT2D.
Encouragingly, similar glycemic outcomes were seen in PwT1D, but PwT2D had more clinically meaningful improvements with fewer sessions each week of OTR mobile app usage than PwT1D (see TABLE).
The data also revealed that the 65 or older age group demonstrated the highest engagement with the app, spending more time on the app than any other age group over the 180-day period. People with diabetes 65 years of age or older (n=7,927 PwT2D; n=775 PwT1D) spent 45 to 50 minutes on the app per week (PwT2D=45 minutes per week; PwT1D=50.9 minutes per week) exhibiting the strongest affinity to using an app to support their diabetes treatment. Additionally, the data showed that the number of hypoglycemic readings were low and fairly similar in both PwT2D and PwT1D aged 65 and older. The real-world evidence showed significant glycemic improvements among app users 65 years of age or older, although the magnitude of improvement was not as high as for the younger age groups. These findings warrant further research about how age and the degree of engagement with a smart blood glucose meter and mobile app affect glycemic outcomes.
Study Methodology
Anonymized glucose and app analytics from two separate datasets were extracted from a LifeScan data lake of more than 55,000 people with diabetes. The first dataset covers 11,583 PwT1D and 45,132 PwT2D using the OTR app with the OTVR meter over a 90-day timeframe, and the second dataset covers 7,107 PwT1D and 32,719 PwT2D over a 180-day timeframe also using the OTR app with the OTVR meter . Data from their first 14 days using the OTR app with the OTVR meter was compared with 14 days prior to 90- and 180-day timepoints using paired within-subject differences.
TABLE
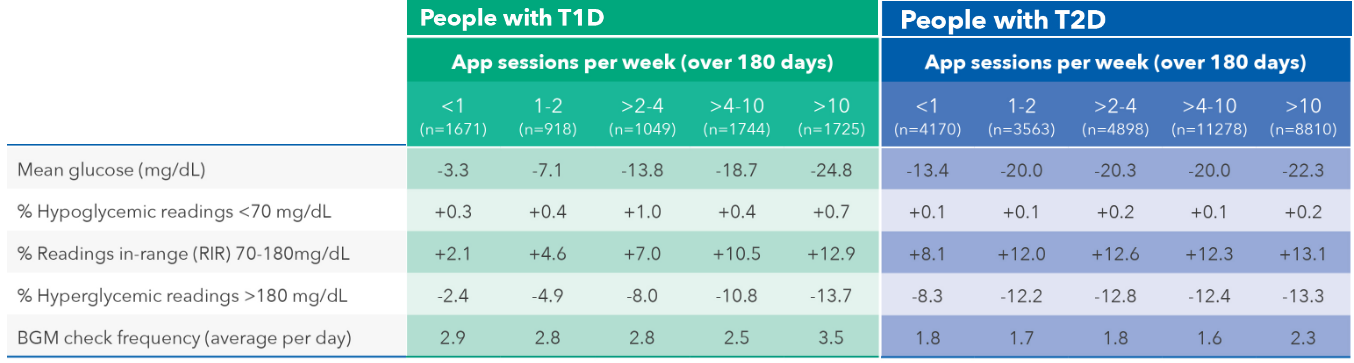
All glycemic changes were statistically significant (P < .005 level).
Abbreviations: PwT1D, people with type 1 diabetes; PwT2D, people with type 2 diabetes.
About the OneTouch® brand made by LifeScan
LifeScan is a global leader in blood glucose monitoring and digital health technology and has a vision to create a world without limits for people with diabetes and related conditions. More than 20 million people and their caregivers around the world count on LifeScan’s OneTouch brand products to manage their diabetes. Together, LifeScan and OneTouch improve the quality of life for people with diabetes with products and digital platforms defined by simplicity, accuracy, and trust. LifeScan.com and OneTouch.com.
The Bluetooth® word mark and logos are registered trademarks owned by Bluetooth SIG, Inc. and any use of such marks by LifeScan Scotland Ltd. and its affiliates is under license.
###